Ideal and Non-ideal Solutions
Ideal and Non-ideal Solutions: Overview
This Topic covers sub-topics such as Azeotropic Mixtures, Ideal Solution, Ideal and Non-ideal solutions, Non-Ideal Solutions, Fractional Distillation of Ideal Solution, Fractional Distillation of Non-Ideal Solutions and, Maximum Boiling Point Azeotropes
Important Questions on Ideal and Non-ideal Solutions
On mixing of with of toulene, the total volume of the solution is
Which of the following are correct for an ideal solution?
a)
b)
c)
d) is an example of ideal solution.
Which of the following is/are "not correct" for mixture solution?
a)
b) Does not obey Raoult's law
c)
d) An example of ideal solution
The number of binary liquids which form ideal solutions among the following are
(1)Solution of benzene & toluene
(2) Solution of hexane & heptane
(3) Solution of chloroform & methanol
(4) Solution of methanol & acetone
(5) Solution of chloroform & acetone
(6) Solution of nitric acid & water
(7) Solution of Aniline & phenol
Which of the mixture of solutions is an example for deviation from Raoult's law?
Identify the correct statement from the following
Identify the correct statement from the following:
Which represents correct difference when non-volatile solute is present in an ideal solution?
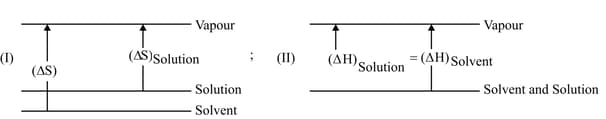
What is azeotropic mixture? Can azeotropic mixture be considered as an ideal solution?
Why does a solution of ethanol and cyclohexane show positive deviation from Raoult's lafw?
Two liquids and on mixing produce a warm solution. Which type of deviation from Raoult's law does it show?
Under what conditions do non-ideal solutions show negative deviations?
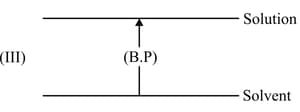
What is the boiling point of an azeotrope of non-ideal solution showing positive deviation as compared to the boiling of its components?
What type of liquids form ideal solutions?
Why pure ethyl alcohol cannot be obtained from rectified spirit even by fractional distillation?
The formation of a solution from two components can be considered as
(i) Pure solvent Separated solvent molecules,
(ii) Pure solute Separated solute molecules,
(iii) Separated solvent and solute molecules Solution,
Solution so formed will be ideal if
Give reason, mixture of ethanol and acetone shows positive deviation from Raoult's law.
Why a mixture of carbon disulphuide and acetone shows positive deviation from Raoult's law ? What type of azeotrope is formed by this mixture ?
